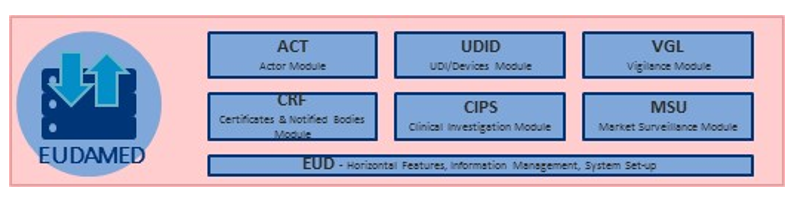
Informatica Experts offers specialist consultancy, training and resource in delivering and implementing compliance of the new EU medical device (e.g. EUMDR 2017/745) regulations across your data systems and processes.
Informatica Experts acknowledges and appreciates that the need to initiate these changes is immediate and overwhelming. We can source and provide the experts, teams and resources required to ensure that your company meets the new regulations. We can provide the workforce that will be able to manage the additional hours, understand the regulations and facilitate the implementation. We can also train your existing staff, leaving you with a sense of reliability that the compliance with the new regulations is fully met.